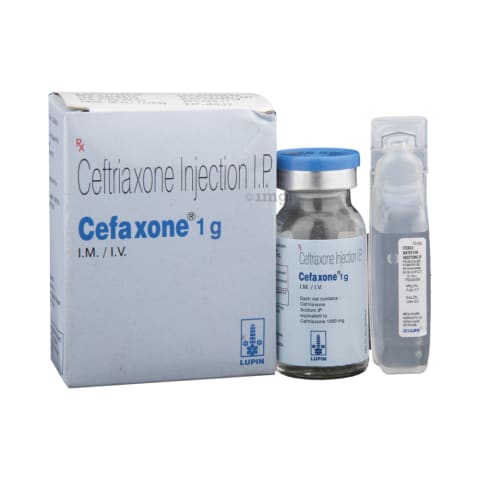
Ceftriaxone Information
Pronunciation
sef trye AKS one
What is this drug used for?
• It is used to treat or prevent bacterial infections.
Frequently reported side effects of this drug
• Injection site irritation
• Diarrhea
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
• Pancreatitis like severe abdominal pain, severe back pain, severe nausea, or vomiting.
• Gallstones like pain in the upper right abdominal area, right shoulder area, or between the shoulder blades; yellow skin; or fever with chills.
• Kidney problems like unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain.
• Hemolytic anemia like severe loss of strength and energy, dark urine, or yellow skin.
• Kidney stone like back pain, abdominal pain, or blood in the urine.
• Seizures
• Clostridioides (formerly Clostridium) difficile-associated diarrhea like abdominal pain or cramps, severe diarrhea or watery stools, or bloody stools.
• Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Medication Safety Issues
Sound-alike/look-alike issues:
CefTRIAXone may be confused with ceFAZolin, cefoTEtan, cefOXitin, cefTAZidime, Cetraxal
Rocephin may be confused with Roferon
Storage and Stability
Powder for injection: Prior to reconstitution, store at ≤25°C (≤77°F). Protect from light.
Premixed solution (manufacturer premixed): Store at -20°C; once thawed, solutions are stable for 48 hours at 25°C (77°F) or for 21 days at 5°C (41°F). Do not refreeze.
Adverse Reactions
Dermatologic: Skin rash, skin tightness (IM)
Gastrointestinal: Diarrhea
Hematologic & oncologic: Eosinophilia, leukopenia, thrombocythemia
Hepatic: Increased serum transaminases
Local: Induration at injection site (incidence higher with IM), pain at injection site, tenderness at injection site, warm sensation at injection site (IM)
Renal: Increased blood urea nitrogen
Rare but important or life-threatening: Abdominal pain, acute generalized exanthematous pustulosis, acute renal failure (post-renal), agranulocytosis, allergic dermatitis, anaphylactoid reaction, anaphylaxis, anemia, basophilia, blood coagulation disorder, bronchospasm, candidiasis, casts in urine, choledocholithiasis, cholelithiasis, clostridium difficile associated diarrhea, colitis, decreased prothrombin time, dysgeusia, dyspepsia, edema, epistaxis, erythema multiforme, fever, flushing, gallbladder sludge, glossitis, glycosuria, granulocytopenia, headache, hematuria, hemolytic anemia, hypersensitivity pneumonitis, increased monocytes, increased serum alkaline phosphatase, increased serum bilirubin, increased serum creatinine, jaundice, kernicterus, leukocytosis, lymphocytopenia, lymphocytosis, nephrolithiasis, neutropenia, oliguria, palpitations, pancreatitis, phlebitis, prolonged prothrombin time, pseudomembranous colitis, seizure, serum sickness, Stevens-Johnson syndrome, stomatitis, thrombocytopenia, toxic epidermal necrolysis, ureteral obstruction, urogenital fungal infection, urolithiasis, vaginitis –